Strategic Consulting
MAC Clinical Research has a long-standing reputation of working closely with our clients to provide a bespoke, personal service. Since 1988, we have provided high quality clinical study services at our wholly-owned research sites; as a result, we have built up a wealth of in-house therapeutic area knowledge. To compliment our clinical research facilities, we offer patient recruitment expertise and complete clinical pharmaceutical research services, including GMP, laboratory facilities, world-class training and endpoint assessments. Combined with our array of scientific solutions, we offer full clinical study management. Our in-house teams at MAC include medical and scientific experts who are frequently approached by current, previous, and prospective clients to consult on matters relating to their clinical research.
MAC Strategic Consulting will support you at any stage of scientific or clinical development, providing a service tailored to your requirements. Our experts offer solutions to address areas where you may lack specific skills, or require additional support with your study design.
MAC is proud to be a consistent supporter of academic research and charitable organisations. For those working in non-profit or academic sectors, we offer a discount on our consultancy services.
If you would like more information or to discuss your specific requirements, please contact us.

MAC offers the following consultancy services:
Our dedicated team of experts works in tandem to provide complete services with a wide range of input and review.
- Study design
- Protocol development
- Non-CTIMP planning, development, analysis, and reporting
- Development of submission-ready publications
- Peer review services across all documentation
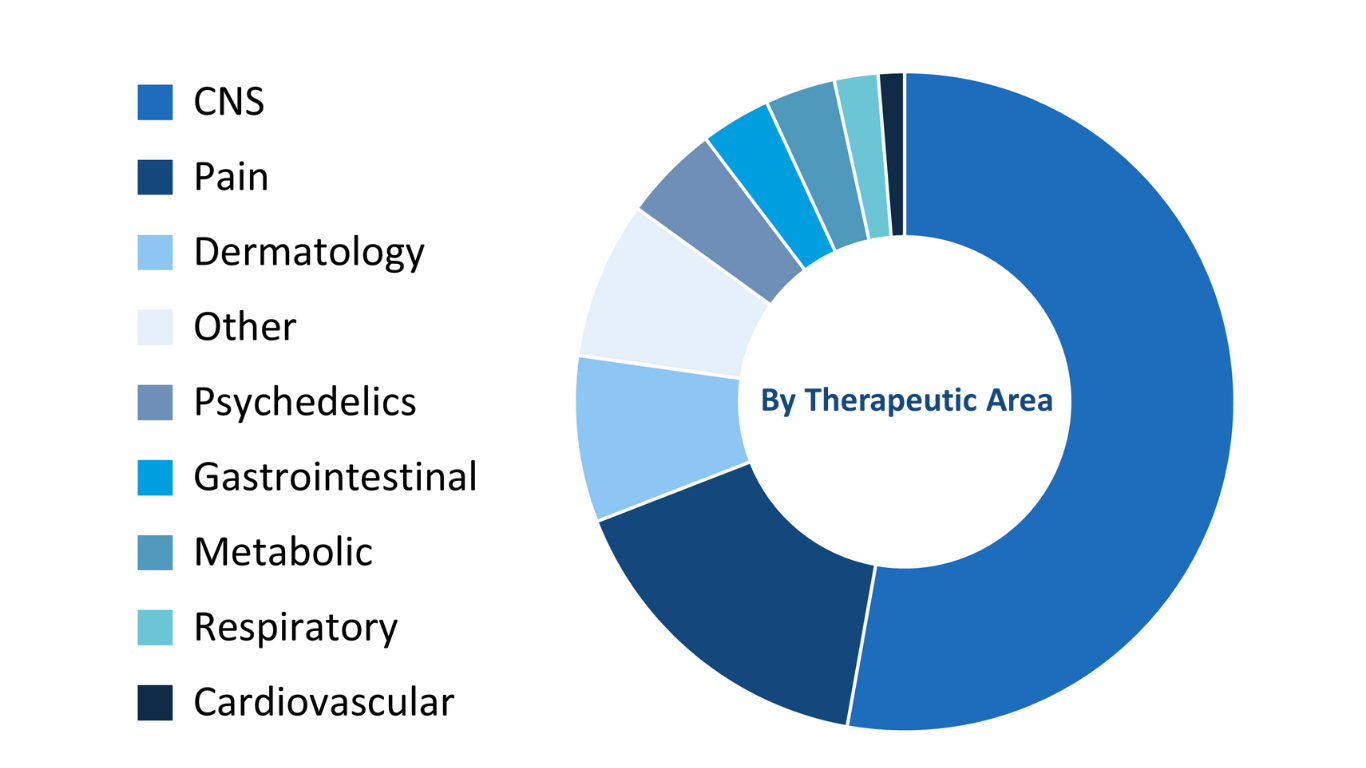
Having facilitated the development and delivery of over 200 clinical trials across the last decade, MAC has extensive experience with a range of therapeutic areas. Our clinical teams and research professionals boast substantial knowledge regarding conducting trials in areas including oncology, psychedelic treatments cognitive and psychiatric disorders, gastrointestinal, metabolic, and respiratory indications, pain, sleep, dermatology, cardiology, and women’s health.
We have dedicated, in-house experts who can offer tailored advice in every therapeutic area to match your research requirements, including:
- Scientific expertise in specific therapeutic areas
- Study recruitment planning with a cross sector approach
- Targeted protocol design tailored to your specific therapeutic area
- Specialised protocol peer review services
- Training programmes for research
- Regulatory submissions and approvals assistance for each targeted area of research, including a masterclass for R&D approval and confirmation of capacity and capability within the NHS
- IMP preparation planning and shipment strategy within clinical trials
- Risk assessment support and safety planning, particularly in relation to patient safety and compliance Technology centred solutions planning within clinical trials
Our Scientific Solutions and Pharmacodynamics group provides key scientific input throughout the drug development process. With science at the heart of everything they do, our teams collectively bring several decades of therapeutic area expertise and will work with you to develop solutions tailored to your specific requirements.
Support includes:
- Scientific and medical expertise in human models of disease
- Production of clinical development plans (CDPs)
- Consultation points, review, and recommendations for serious adverse events (SAEs) and adverse events (AEs)
- Protocol design/development across early- and late-phase trials, covering areas such human models of disease and methods/techniques in cognitive and neuropsychological assessments
- Training programmes for scientific services, placebo, and endpoints assessments
- Regulatory submissions and approvals required in scientific research for ethics committees (ECs)
- Risk assessment support and safety planning, particularly in relation to patient safety and compliance with regulations such as IQIPS (for which MAC’s Liverpool sleep lab is UKAS-accredited)
- Neurophysiology expertise in NHS and Clinical Trials for Techniques, such as EEG, nerve conduction, and sleep assessments
- Cognitive and neuropsychological assessments expertise
- Expertise, resources, and requirements for equipment and methodology in clinical trials
- Data collection and eCRF structure advice
- Technology-centred solutions planning within Scientific Services for clinical trials
Our Clinical Science and Development team has been part of the pharmaceutical and biotechnology industry for over two decades, with special interest and extensive experience in pre-clinical early- and late-phase developments, including registrations studies for MAA and NDA approvals. Our team comprise members with extensive CRO and pharma experience.
They will work hand in hand with you to support your development needs and offer:
- Extensive early- and late-phase and regulatory expertise in a wide range of clinical specialities
- Assistance with clients to develop pre-clinical and clinical development and regulatory pathways tailored to their drug and indications.
- Modular Integrated Development Plans (IDPs) with go/no-go and risk mitigation strategies
- Tailored industry guidance on study designs and endpoints in accordance with local and national regulatory requirements
- Clinical science support through all stages of pre-clinical and clinical development
- Development of Target Product Profile (TPP)
- Expertise in Common Technical Documents (CTDs) for Market Authorisation Applications (MAA) and New Drug Applications (NDAs)
- Key opinion leader (KOL) development in different therapeutic areas
MAC has a dedicated team of medical experts of who come from a wide range of clinical backgrounds. This team has several decades of extensive experience contributing their breadth and depth expert medical knowledge, having acted as the Chief and Principal Investigators on early- and late-phase studies. Depending on a study’s requirements and therapeutic area, our team will identify the relevant expert to provide you with support suited to your needs, including:
- Scientific and medical advice
- Therapy area analysis and asset positioning
- Clinical development plans (CDPs)
- Target patient population (TPP) and eligibility criteria
- Protocol design, including adaptive and umbrella style studies
- Project risk assessment, management, and mitigation strategies
- Safety overview and adverse event review and monitoring
- Dose escalation, SRC, and DLRC representation
- Recruitment and enrolment strategies
- Regulatory submissions and ethics and CTA applications
- CSR review and input
Our team of statisticians have wide-ranging experience from both academia and industry, and will work as part of your team to plan, execute, and report all analyses according to your specific requirements. With extensive experience in SAS, R, and Pheonix WinNonlin, our team can adapt to your needs and work within any research group to provide expertise or training tailored to you, including:
- Statistical and scientific advice
- Analysis planning and interpretation
- Statistical Analysis Plan (SAP)
- Study design, including adaptive design
- Protocol design
- Post-hoc analysis
- Tailored reporting and analysis
- Statistical reporting
- Meta-Analysis
- Pharmacokinetic/pharmacodynamic analyses
- New drug applications (NDAs)
- Standard and complex statistical analysis, including MI, MMRM, and simulation techniques
Our team of medical writers have a wealth of experience contributing to and creating study documentation and reports across a broad range therapeutic areas. Our team will work with you, as needed, to develop specialised templates and documentation suited to your requirements, including:
- Protocol design
- Ad-hoc reports
- Publication support
- ICF/PIS development
- CSR development/support
Our Laboratory Services Department is comprised of 3 separate groups: Clinical Pathology, Sample Receiving & Preparation, and Bioanalytical Services. Our staff have accumulated decades of specialised knowledge in their respective scientific disciplines. With extensive experience across different techniques and therapeutic areas, the team will work closely with you to create a service suited to your needs, including:
- Instrument selection
- Lab design
- Method transfer
- Validation/verification of methods
- Regulatory compliance
- Clinical research training
- CPD programs/courses
- Case study review
- Clinical trials virtual management