Taking a new treatment from lab to patient is a long, highly regulated process that demands years of research, significant investment and close collaboration between scientists, clinicians, Sponsors and regulators. At the core of this process are clinical trials.
These trials unfold in four key phases, each one designed to answer a different set of questions. Starting with basic safety and dosing and progressing through to long-term efficacy and how the treatment performs in everyday clinical settings. Every phase is critical to the overall success of a new treatment, and each brings its own scientific, logistical and operational challenges.
Knowing what to expect at each stage can make a real difference. Whether you’re planning a study or partnering with a contract research organisation (CRO), a solid understanding of the clinical trial pathway will help you make better decisions, avoid common mistakes and stay a step ahead.
Phase I
Phase I trials mark the first time a new drug or therapy is tested in humans. The focus here is on safety: researchers are looking to understand how the treatment behaves in the body, what doses are safe, and what side effects might occur. These studies are usually small, often fewer than 100 participants, and involve either healthy volunteers or patients, depending on the treatment. Despite their size Phase I trials can be incredibly challenging.
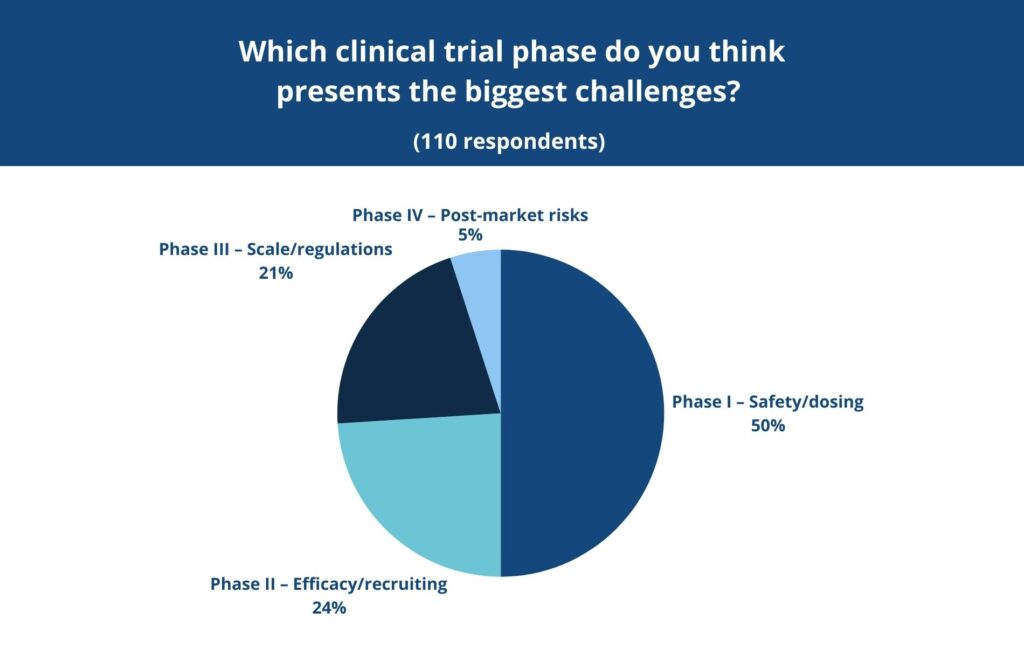
In a recent LinkedIn poll conducted by MAC, 50% of respondents said they found Phase I to be the most difficult stage of the clinical trial process. This could relate to the uncertainty at this early stage, the intensity of regulatory oversight and the need for meticulous monitoring in controlled environments.
Phase II
Phase II shifts the focus to efficacy – does the treatment actually work for the condition it’s intended to treat? These studies are larger and involve participants who have the condition in question. Researchers also continue to monitor safety and adjust dosing. Recruitment becomes a major factor here and it’s not always easy to find the right patients, at the right time, who meet strict eligibility criteria. In the same poll mentioned earlier, 24% of respondents pointed to Phase II as the most challenging. This most likely reflects the logistical and scientific hurdles that come with trying to prove a treatment’s effectiveness for the first time.
Phase III
By the time a treatment reaches Phase III, the stakes are even higher. These large-scale studies, often involving thousands of participants across multiple sites and countries, are designed to confirm efficacy, monitor side effects and compare the new treatment with standard therapies. A successful Phase III trial is usually the final step before regulatory submission. However, coordinating across so many sites and meeting increasingly complex regulatory requirements is extremely difficult. In our poll, 21% of respondents named Phase III as the most difficult phase, citing the enormous operational and compliance pressures that come with studies at this scale.

Phase IV
Even after a drug is approved the work doesn’t stop. Phase IV trials, often called post-marketing studies, look at how a drug performs in the real world, outside the controlled environment of earlier trial phases. These studies are important for picking up on long-term or rare side effects that might not have been visible during earlier stages, and they can lead to updates in dosing, safety information or clinical guidelines.
Interestingly, only 5% of respondents in our recent poll said Phase IV was the most challenging part of the clinical trial process. That might be because fewer people have direct experience with this phase as, after all, only about 12% of medicines that enter clinical research ever make it all the way to market (1).
Each phase presents its own set of hurdles and for many Sponsors, especially those without large internal research teams, navigating them can be overwhelming.
Partnering with the Right CRO
This is where partnering with the right CRO can make a huge difference. At MAC, we have over 30 years of experience in the medical research industry. Because we own and operate all our clinical trial sites across the UK, we have full oversight of recruitment, conduct, and data quality. This level of control not only enables faster start-up times and more consistent patient engagement – it also allows us to develop and refine robust procedures that we can implement and standardise across our global network of partner sites. By applying the same high standards internationally, we help ensure greater consistency and confidence in study delivery, wherever a trial is being run.
Our in-house recruitment team, data management specialists and regulatory experts enable us to deliver a fully integrated approach, with Sponsors benefiting from a single, experienced partner managing everything from protocol design and ethics submissions to statistical analysis and final reporting. Our infrastructure and hands-on approach allow our Sponsors to stay focused on what matters most: advancing treatments that improve lives.
Understanding each phase of the clinical trial process isn’t just about knowing what comes next. It’s about anticipating challenges, building in flexibility, and working with partners who can deliver. With MAC, that experience and capability is built in from the start.
Contact us to find out how we can help support your study here.











