Clinical Operations
Site Monitoring
MAC’s Clinical Research Associates (CRAs) ensure the highest quality review of data and optimal interaction with all study sites, for both blinded and unblinded studies. Our clinical monitoring and site management teams are experienced in managing all clinical trial types and sizes, regardless of complexity.
Services include:
- Site evaluation and selection
- Site feasibility assessments
- Budget insight
- Contract management
- Site monitoring and management
- Remote site and study monitoring via EDC
- 100% targeted or risk-based monitoring
- Regulatory tasks and related essential documentation management
- Electronic trial master file (eTMF) management
- Management and facilitation of institutional review board (IRB) and/or ethics committee (EC) submissions
- Study and site closeout

CRA Focus
Our CRAs work independently and proactively, with a key focus on consistent alignment with the compliance principles for study quality and delivery specified by the US Federal Department of Agriculture (FDA), the International Council for Harmonisation’s Good Clinical Practice (ICHGCP), and the UK’s Medicines for Human Use (in Clinical Trials) Regulations. These principles state that all study deliverables must be : attributable, legible, contemporaneous, original, and accurate (ALCOA), .
MAC’s CRAs consistently focus on project delivery that ensures:
- Verification and protection of our human study subjects’ rights and wellbeing
- Accurate, complete, and verifiable source data documentation
- Study conduct follows approved protocol amendments and all applicable regulatory requirements
Lead CRAs are the primary point of contact between the CRA and Project Management teams. Study roles and responsibilities include the following aspects of Phase I-IV clinical trials:
- Review of clinical monitoring, including risk-based, centralized, remote, and on-site, as well as clinical monitoring reports
- Risk management and document development
- Clinical trials processes including feasibility, site initiation, and study conduct and close-out
- Clinical monitoring activities throughout the study
- Document management
- Data management
- Investigational medical product (IMP) management and drug development process
- Vendor management
- Management of all inspections and audits by the Medicines and Healthcare Regulatory Agency (MHRA) and FDA
MAC offers the following monitoring models:
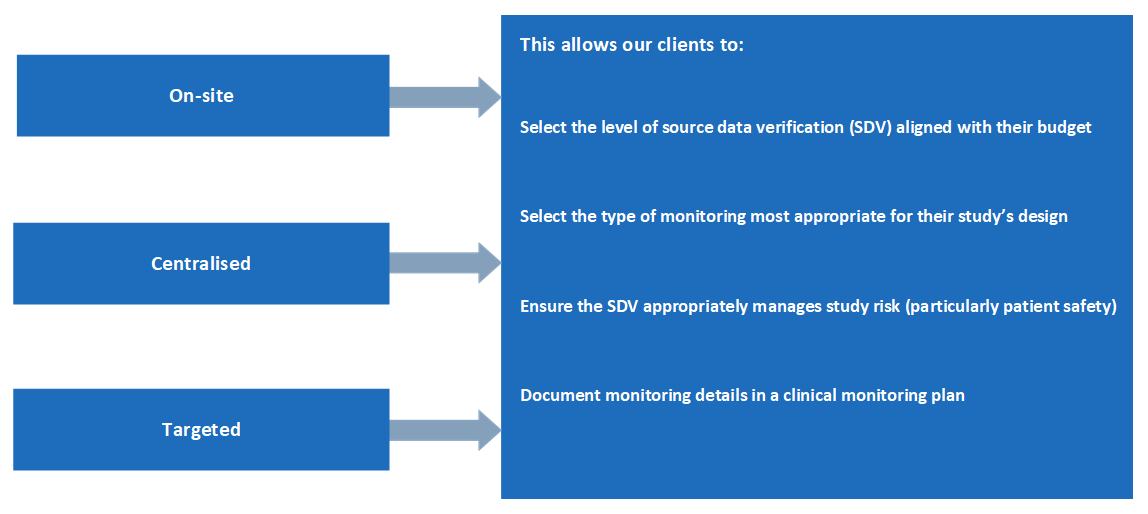
Site Management
MAC understands that successful study delivery starts with an accurate feasibility assessment, leading to selection of high performing sites. Our independent clinical operations group provides quality site management services, managing the full site lifecycle from selection through to closeout for every stage of your clinical trial. MAC is pleased to offer both on-site and remote SDV models; our expertise allows us to offer the most strategic and efficient site management approach for your study.







