Clinical Assessments and Procedures
Scientific Services and Endpoint Assessments
Clinical Assessments
Scientists at MAC have extensive experience in the set-up, validation and execution of a wide range of scientific assessments and techniques. These methods can be applied in early or late phase clinical research.
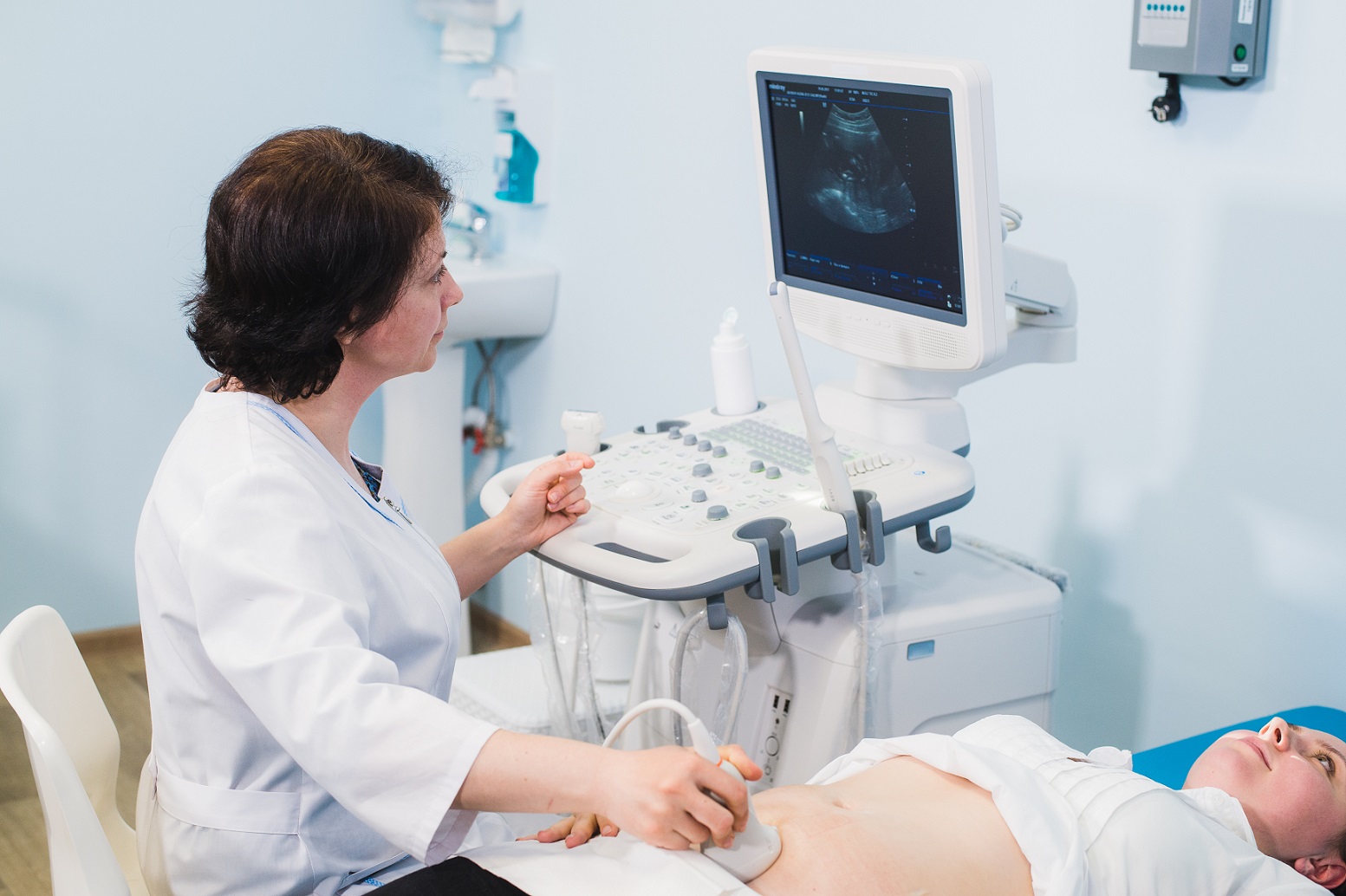
MAC Clinical Research provides a range of scientific assessments:
- Automated office blood pressure (AOBP)
- Home blood pressure monitoring (HBPM)
- DXA scan (dual-energy X-ray absorptiometry): fat mass measurement in correlation with CVD events/risks
- Echocardiogram (ultrasound of the heart)
- Blood tests (e.g. cholesterol/triglycerides, BNP)
- 6MWT (6-minute walk test)
- Telemetry
- 24-hour blood pressure holter (wearable)
- 24-hour blood pressure assessments
- Electrocardiogram (ECG) assessments
- QT and QT-waiver studies
Standardised areas of clinical and cognitive rating assessments include:
- Addiction
- Agitation
- Aggression
- Anxiety (Generalised Anxiety Disorder, Social Anxiety)
- Autism Spectrum Disorder (ASD)
- Bipolar Disorder I and II
- Cognition
- Delirium
- Dementia
- Eating disorders (Anorexia Nervosa, Bulimia Nervosa, Binge Eating Disorder)
- Major Depressive Disorder (MDD)
- Obsessive-Compulsive Disorder (OCD)
- Panic Disorder
- Post-Traumatic Stress Disorder (PTSD)
- Sleep Assessments
- Suicidality
- Patient-Reported Outcomes (PROs)
Our dedicated rating team, comprised of psychiatrists, clinical psychologists, specialist nurses, and therapists, use recognised and gold-standard testing along with their own clinical assessment experience for initial screening of participants, using assessments such as: Structured Clinical Interview for DSM-V (SCID), Mini-International Neuropsychiatric Interview (MINI) and rating scales, including but not limited to: MMSE, HDRS, CSSRS, SAPS, CDR, HTVL-R, WMS-IV, MADRS, CGI-I, CGI-S, GCAS, SIGMA, HAMD-17, HAM-A, SIGH-D/A, GDS, KSS, EQ-5D-5L, ZBI, BPST-D, GCAS, NIP, FCSRT, AMT, Ishihara Colour Test, GDS, Sentence Completion Test, ABS, IQ tests, CAT, CAPs, HRS CANTAB, CogState.
- Skin rating scales (SCORAD, EASI, vIGA-AD, PASI, BSA, PGA)
- Skin-punch biopsies
- Ultrasound
- Laser Doppler
- Functional magnetic resonance imaging (fMRI)
- Positron emission tomography (PET)
- High-resolution computed tomography (HRCT)
- Magnetic resonance spectroscopy (MRS)
- Nuclear medicine
- Laser-guided injection
- Intra-articular temperature probes
- Electroencephalogram (EEG):
- Routine
- Sleep-Deprived
- Ambulatory
- Quantitative
- Sleep assessments:
- Polysomnography
- (PSG)
- Multiple Sleep Latency Test (MSLT)
- Maintenance of Wakefulness Test (MWT)
- Actigraphy
- Electromyography
- Nerve conduction studies
- Microneurography
- Evoked potentials
- Quantitative sensory testing
- Thermal thresholds/tolerance
- Pressure pain thresholds/tolerance
- Stimulus-induced pain
- Pain matching
- Hyperalgesia/allodynia
- Nerve conduction
- Saccadic eye movements (oculography)
- Pupillometry
- Postural stability
- Respiratory function testing
- Bleeding time
- ExNO
- Platelet aggregometry
- RigiScan
- Minor starch iodine test
- PH monitoring
- Peak-flow
- Flow loops
- Transfer factors in respiratory
- Infrared skin temperature
- pH monitoring
- Pulmonary function tests
Clinical Procedures
At MAC, we specialise in a range of advanced clinical, minimally invasive surgical, and non-surgical procedures, all performed to the highest standards of safety and quality. We strictly adhere to our standardized protocols and benefit from the strong collaboration of external consultant specialists. Additionally, our skilled laboratory personnel are highly experienced in sample processing, fixation, and shipment, ensuring continuous and efficient support throughout each procedure. Patient safety and care are our top priorities.
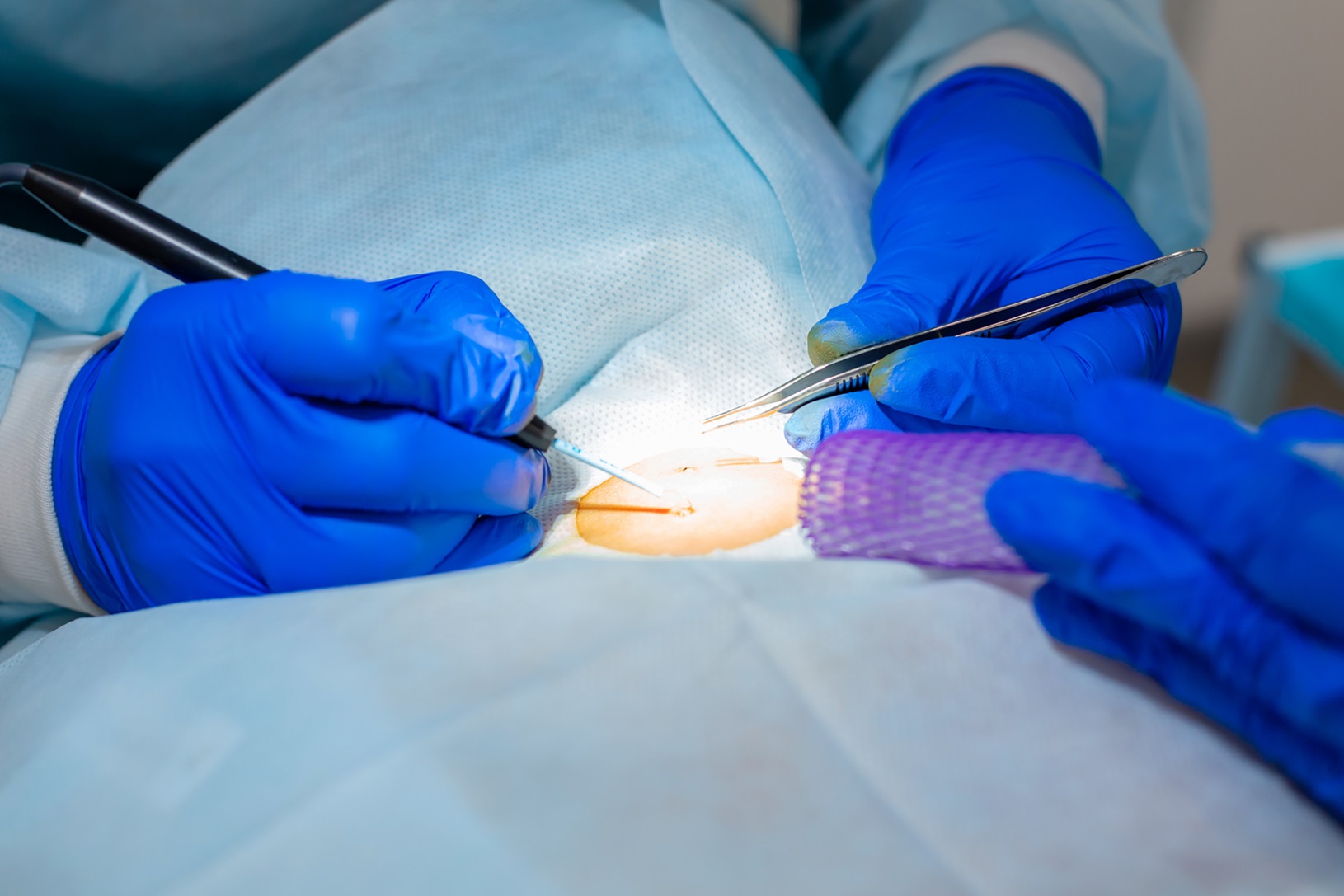
MAC Clinical Research provides a range of procedures:
A skin punch biopsy is a minimally invasive procedure where a small sample of skin tissue is removed using a punch tool under local anesthesia. At MAC, we primarily perform this procedure for investigational purposes in our clinical trials to identify biomarkers, monitor microscopic skin changes, and evaluate the efficacy of investigational products. With minimal downtime and rapid recovery, this technique provides crucial diagnostic information while ensuring patient comfort and care.
Muscle biopsies can be performed using either an open surgical approach or a less invasive percutaneous technique. At MAC, these biopsies are performed mainly for research purposes, helping to identify specific biomarkers and assess the stage of neuromuscular or metabolic conditions in clinical trials. The open biopsy involves surgically removing a small segment of muscle, while the percutaneous method uses a needle to extract a sample, reducing recovery time. Both approaches provide valuable insights that aid in the evaluation of investigational treatments, all while upholding the highest standards of patient safety.
An ultrasound-guided liver biopsy is a precise procedure utilized to obtain tissue samples to identify biomarkers and assess liver conditions like NASH, cirrhosis, or cancer in the context of clinical research. The needle is guided by ultrasound to ensure accuracy and safety. At MAC, this procedure allows us to monitor the progression of liver damage and fibrosis, as well as the efficacy of investigational products, all while prioritizing patient care through vigilant post-procedure monitoring.
Arthrocentesis, or joint aspiration, is a minimally invasive procedure performed to collect synovial fluid from a joint, typically the knee, under sterile conditions. In our clinical trials, this procedure is crucial for identifying biomarkers and assessing joint conditions such as infections, gout, or inflammatory arthritis, while also monitoring the efficacy of investigational treatments. By providing quick and reliable samples, arthrocentesis aids in targeted therapeutic decisions while minimizing patient discomfort.
A lumbar puncture, commonly known as a spinal tap, involves the insertion of a thin needle into the lower spine to collect cerebrospinal fluid (CSF). At MAC, we perform this procedure primarily for investigational purposes in our clinical trials to identify neurological biomarkers, assess disease stages, and monitor the effectiveness of investigational products. The procedure is conducted with utmost precision to ensure patient safety and comfort, facilitating detailed analysis and informed treatment strategies.







