Metabolomics
What is Metabolomics
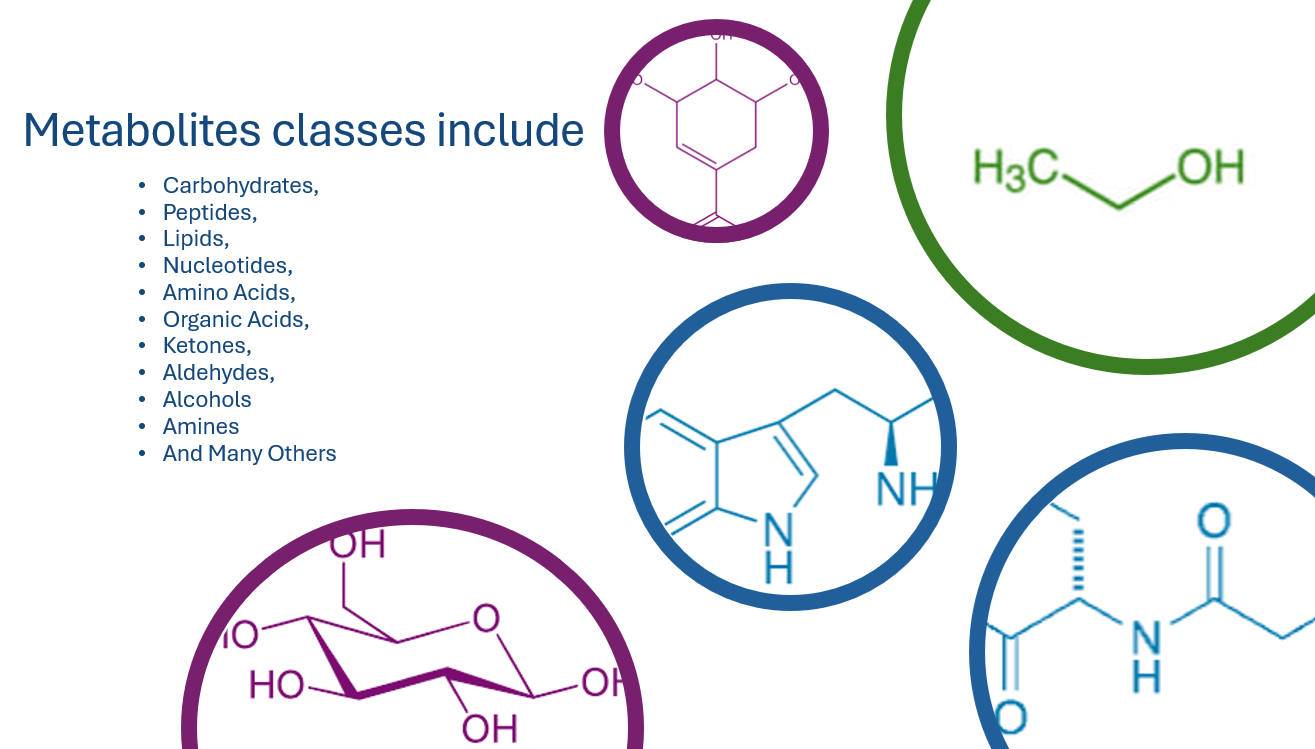
Based on over 180 years of research into biochemistry and metabolism, metabolomics is the measurement of as many of the metabolites of a living organism as possible. There are a large number of chemical classes with a wide range of concentrations. Commonly, metabolomics is a comparative analysis using mass spectrometry coupled and statistics. Metabolomics is more than just an analytical chemistry problem; it requires a multidisciplinary team with expertise in chemistry, biochemistry, bioinformatics, statistics and a global understanding of the living system.
Type of Metabolomics
Targeted
• Quantitative
• Uses authentic standards to measure the concentration of a finite number of metabolites
• Easy to associate with biochemical pathways
• Methods easily transferable
Hybrid
• Quantitative & Semi Quantitative
• Uses authentic standards to measure a finite number of metabolites whilst still collecting non-target data
• Known metabolites easy to associate with biochemical pathways
Non-Targeted
• Semi Quantitative
• The coverage of the non-target data is dependent on the analytical technique used
• Relies on pattern matching and statistical analysis to highlight features of interest
• There is a large amount of work to identified features of interest if needed
Metabolomics, the systematic study of small molecules or metabolites in a biological system, has emerged as a powerful tool in biomedical research.
By providing insights into the metabolic pathways and cellular processes underlying disease, metabolomics offers unique opportunities to advance clinical trials and improve patient outcomes.
Disease-Specific Metabolites: Metabolomics can identify unique metabolic signatures associated with specific diseases, aiding in early diagnosis and prognosis.
Mechanism-Based Biomarkers: By understanding the metabolic pathways involved in disease pathogenesis, metabolomics can discover biomarkers that reflect disease mechanisms and treatment response.
Companion Diagnostics: Metabolomics can contribute to the development of companion diagnostics, which can help identify patients most likely to benefit from a particular treatment.
Target Identification and Validation: Metabolomics can identify potential drug targets by revealing metabolic pathways that are dysregulated in disease.
Drug Efficacy and Safety: Metabolomics can assess drug efficacy by monitoring changes in metabolic profiles associated with disease progression or remission.
Additionally, it can help identify potential adverse drug reactions by detecting metabolic perturbations.
Mechanism of Action: Metabolomics can elucidate the mechanisms of action of new drugs by studying their effects on metabolic pathways.
Subpopulations: Metabolomics can identify distinct subpopulations of patients with different metabolic profiles and disease prognoses, allowing for tailored treatment strategies.
Predicting Treatment Response: By analysing metabolic biomarkers, it is possible to predict which patients are more likely to respond to a particular treatment, reducing the risk of unnecessary exposure to ineffective therapies.
Tailored Treatments: Metabolomics can inform the selection of optimal treatments for individual patients based on their unique metabolic profiles.
Monitoring Treatment Response: Metabolomics can be used to monitor treatment response in real-time, allowing for adjustments to the treatment plan as needed.
Endpoint Selection: Metabolomics can help identify relevant endpoints for clinical trials, ensuring that the studies measure meaningful outcomes.
Patient Enrolment: Metabolomics can be used to select patients for clinical trials based on their likelihood of benefiting from the intervention.
Trial Monitoring: Metabolomics can provide real-time data on patient safety and efficacy, enabling early detection of adverse events and adjustments to the trial design.
Data Analysis: The analysis of metabolomics data can be complex and requires specialised bioinformatics tools.
Standardisation: Efforts are underway to standardize metabolomics methods and data analysis pipelines to ensure reproducibility and comparability across studies.
Integration with Other Omics: Integrating metabolomics data with genomics, transcriptomics, and proteomics can provide a more comprehensive understanding of disease biology.
The Benefits of Metabolomics in Clinical Trials
Metabolomics has the potential to revolutionise clinical trials by providing valuable insights into disease mechanisms, drug development, patient stratification, and personalized medicine. As the field continues to advance, metabolomics will play an increasingly important role in improving patient outcomes and accelerating the development of effective therapies. This could increase the efficiency of clinical trial by reducing costs, improving outcomes and shortening durations.
- Improved safety: By using metabolomic to stratify populations and monitor for SEA, the participants risk profile can be reduced.
- Improved Data quality: Metabolomics provides information rich data source that allow a greater insight into how a drug is interacting with a person and a population.
- Increased efficiency: Stratifying the test population means that more meaningful data can be generated more rapidly.
- Reduced cost: By selecting the correct population the statistical rigor can be improved leading to clearer outcomes.
- Greater understanding: The increase in the breadth and depth of data gives a greater insight into how a drug is interacting with the target and non-targets alike.